
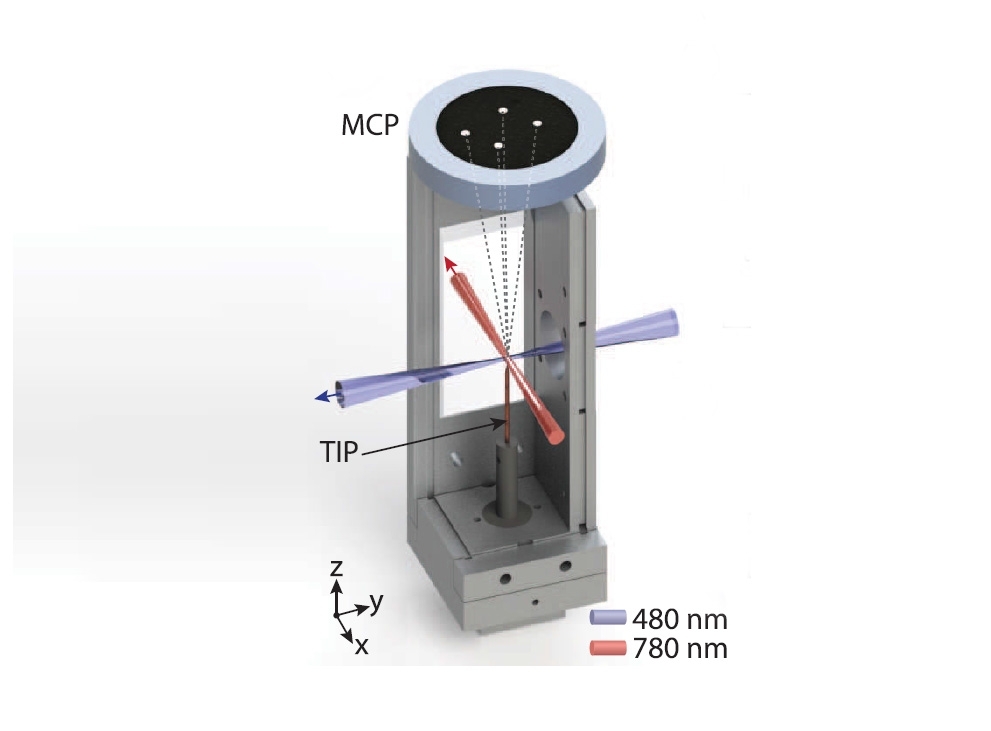
Inside an ultra-high-vacuum chamber, atoms are captured in a magneto-optic trap and intensely excited by a laser pulse. They then receive a very strong electric pulse from the tip of a needle electrode and are driven against a detector (MCP), resulting in a sequence of images (image: detail of illustration from an article published in Physical Review Letters)
The study, in which a Brazilian PhD student took part, involved an experimental calculation of the interaction constant between atoms, confirming the theoretical value.
The study, in which a Brazilian PhD student took part, involved an experimental calculation of the interaction constant between atoms, confirming the theoretical value.

Inside an ultra-high-vacuum chamber, atoms are captured in a magneto-optic trap and intensely excited by a laser pulse. They then receive a very strong electric pulse from the tip of a needle electrode and are driven against a detector (MCP), resulting in a sequence of images (image: detail of illustration from an article published in Physical Review Letters)
By José Tadeu Arantes | Agência FAPESP – An experiment conducted at the University of Michigan’s Physics Department in the United States has monitored the trajectories of individual atoms and recorded images of them. The study was performed by Brazilian PhD student Luís Felipe Gonçalves, his Thai colleague Nithiwadee Thaicharoen, and their supervisor Georg Raithel. An article entitled “Atom-Pair Kinetics with Strong Electric-Dipole Interactions” describing their findings and signed by all three researchers has been published in the journal Physical Review Letters.
Gonçalves is concluding his doctoral work at the University of São Paulo’s São Carlos Physics Institute (IFSC-USP) in Brazil, with a scholarship from FAPESP. Previously, he was a research intern at Michigan, also with a scholarship from FAPESP.
A noteworthy outcome of the study was the experimental measurement of the numerical value of the dipolar interaction between two atoms using atomic distribution imaging. This parameter describes the variation in an atom’s energy as a function of its distance from a neighboring atom. For the chosen material, rubidium (Rb) in the excited state, the theoretical value in joules and cubic meter (J.m3) is 3.72 x 10-42. The value determined in the experiment was (3.3 ± 1.8) x 10-42.
“It was a direct measurement of the parameter,” Gonçalves told Agência FAPESP. “It was also the first time this interaction between two atoms had ever been imaged. We observed experimentally that the interaction is in fact anisotropic, meaning it depends on the relative positions of the atoms.”
The experiment was performed with rubidium atoms in an ultra-high-vacuum chamber. Captured in a magneto-optic trap comprising three orthogonal laser beams and an external magnetic field, tens of millions of atoms in the fundamental state were held in a spherical region with a diameter of approximately 1 cm at the intersection of the three beams.
In this cloud of tens of millions of atoms, a much smaller number were cooled by laser pulses from the fundamental state to what is known as the Rydberg state. Named for Swedish physicist Janne Rydberg (1854-1919), this highly excited state corresponds to a situation in which the electrons in an atom are pushed by an external burst of energy to the layers farthest from the nucleus without becoming detached from the atom. Up to this point, therefore, no ionization occurs.
“In the excited state, these atoms become highly interactive,” Gonçalves said. “And in the highly specific state known as the ‘50S Rydberg state,’ their interaction is isotropic and repulsive. Simply put, this happens because the electrons farthest from the nucleus spread out with equal probability in all directions. Owing to the electromagnetic repulsion caused by the electrons’ negative charge, the atoms repel each other, but they do so isotropically, meaning the repulsion does not depend on direction. To advance the experiment, the next step was to apply an electrical field to the ensemble.”
The external electric field polarized each excited atom, causing the most distant electrons to cluster with greater probability in a certain region of the outer layer. Thus, although the atom remained electrically neutral overall because of the balance between the positive charge of the nucleus and the negative charge of the electrons, its interior now behaved like an electric dipole – not unlike a small magnet, with the nucleus as the positive pole and the cloud of electrons as the negative pole.
“We took care to increase the intensity of the electric field very gradually so as to produce an adiabatic transformation and avoid a change in the atomic state,” Gonçalves said. An adiabatic process is one in which no heat is gained or lost by the system.
Once polarized, the atoms interacted anisotropically, attracting or repelling each other according to their relative positions, i.e., according to the angle between the direction of the external electric field and the internuclear axis. “When the atoms’ polarization axis, which has the same direction and orientation as the electric field, aligns with the internuclear axis, they attract each other. And they repel each other when the field is applied in the orthogonal orientation,” Gonçalves said.
Propelled by electrical attraction or repulsion, the atoms inside the trap evolved over time, until the moment at which they were ionized by a very strong electric pulse from the tip of a needle electrode located some 400 microns below them. The farthest electrons were torn off, and the resulting ions were driven against a detector. “This detector has a phosphor screen that fluoresces whenever an ion impinges on it,” Gonçalves said. “So sequences of images were generated showing the position of each atom.”
An ion beam spreads out on firing, so that the distance between two atoms increases, widening from a few microns in the trap to millimeters as they hit the detector. This difference is controlled and can be measured. “We measured the distances between all ions, two by two, for each firing and obtained a set of values for the x and y coordinates,” Gonçalves said. “We converted each pair of values into a dot in a two-dimensional correlation matrix. Each frame in the matrix corresponded to roughly 5,000 images recorded by the detector. Successive frames, generated at two-microsecond intervals, enabled us to observe the evolution of the interactions over time.”
The difference in pattern between the frames that were generated when the ions were attracting or repelling each other showed that the interaction was indeed anisotropic. This was the first time any images had been obtained of this effect at the atomic level. By measuring the evolution of the distances, the researchers were able to calculate the actual value of the interaction parameter, confirming the theoretical value. Analogously to what happens when iron filings are scattered on a sheet of paper and a magnetized bar is placed under the sheet so that the lines of force in the magnetic field can be seen, the far more sophisticated procedures used in this experiment can be said to have permitted visualization of the spatial organization of particles due to the application of an external electric field.
In this animation, only the repulsive aspect of the interaction is shown. Increasing interatomic distances are reflected by the growing diameter of the disc. The interaction parameter can be extracted from the sequence (animation produced by the researcher).
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.




