
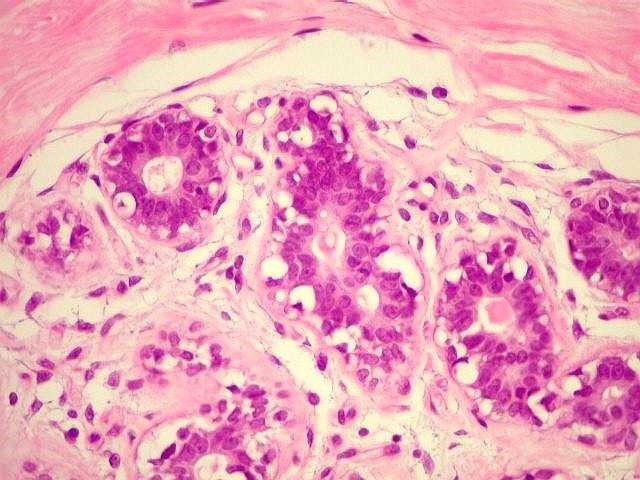
Mina Bissell of the Lawrence Berkeley National Laboratory gathers evidence to show that tissue architecture is as important as changes in DNA for tumor growth (image: Unicamp)
Mina Bissell of the Lawrence Berkeley National Laboratory gathers evidence to show that tissue architecture is as important as changes in DNA for tumor growth.
Mina Bissell of the Lawrence Berkeley National Laboratory gathers evidence to show that tissue architecture is as important as changes in DNA for tumor growth.

Mina Bissell of the Lawrence Berkeley National Laboratory gathers evidence to show that tissue architecture is as important as changes in DNA for tumor growth (image: Unicamp)
Agência FAPESP – Cancer has long been viewed as a disease that is basically genetic, or, in other words, caused by mutations in DNA – inherited or acquired – that alter gene expression and cause cells to proliferate uncontrollably.
However, Iranian-born U.S. scientist Mina Bissell, an exponent of breast cancer study, posits that this is only part of the story. According to her, half of the factors needed for tumor development are found outside of the cells, in what is known as the cellular microenvironment.
“If the genome were really the dominant factor, a single inherited mutation would be enough to cause cancer all over the body, as long as all the cells share the exact same DNA,” Bissell said during a lecture delivered on September 2, 2014, at the Institute of Biomedical Sciences of the University of São Paulo (ICB-USP).
For the past 30 years, the researcher, who currently heads a laboratory that bears her name at the Lawrence Berkeley National Laboratory in the U.S., has gathered evidence to prove her theory that the form and function of a particular tissue are regulated reciprocally, in a dynamic way, and that any alteration of the architecture and signaling network can result in malignancy.
Her research studies have already generated nearly 380 articles published in high-impact journals. Some of the major findings were presented to the Brazilian public during the lecture at USP.
“We selected the mammary gland as a model to study because it is one of the few tissues that change during adult life. It grows during pregnancy and nursing, and when nursing is discontinued, the gland reverts back to its previous size,” Bissell said.
To investigate how these alterations in tissue occur, the researcher concentrated on structures known as acinus, or small sacs found in the breast whose walls are coated with cells specialized in the secretion of milk.
“We removed these structures from pregnant female mice and placed them in an in vitro culture to see if they would remember how to be a mammary gland. However, before long, they assumed a completely different structure and forgot how to make milk. This shows that it is the microenvironment that tells the cells what they should do. Cells are not autonomous, as some biologists continue to believe,” the researcher explained.
What, after all, is this microenvironment? According to Bissell, it is what is known as the extracellular matrix, or a mass that connects cells and is composed of molecules such as collagen, glycoproteins, integrins and laminin.
Back in the 1980s, Bissell formulated the theory of dynamic reciprocity, according to which the extracellular matrix sends signals to the cell nucleus that result in a remodeling of the chromatin and a change in gene expression. The nucleus then signals back, causing remodeling of the extracellular matrix. Form and function thus regulate each other reciprocally.
To test the hypothesis of the existence of a type of communication between cells and their microenvironment, Bissell reproduced the experiment using the mammary cells of female mice, but this time, she placed them on a gel containing some of the main components of the extracellular matrix. Instead of assuming the flattened, two-dimensional structure that they had in the first experiment, the cells organized themselves in a similar way to that observed in vivo and, what was more surprising, continued to secrete milk.
This three-dimensional cell model of the mammary gland was adapted to create a test capable of differentiating a normal cell from a malignant cell. To do this, Bissell and her colleagues used a series of human cell lineages (HMT3522) from a healthy patient undergoing breast reduction surgery.
When cultivated for 10 days in a laminin-rich three-dimensional environment, these cells were capable of recapitulating the features of the normal mammary gland and presented a cell cycle pattern of controlled and established proliferation.
“We placed one of these lineages in a plate and removed epidermal growth factor [a protein important for normal development of the mammary gland]. The transformed cells began to rapidly proliferate, and when injected into an animal, it was possible to form a tumor,” Bissell explained.
When the malignant cells were compared with normal cells in a regular two-dimensional culture, they appeared exactly the same. However, when placed in a 3D model, the malignant cells assumed the disorganized features of a tumor.
“The architecture and beauty of the tissue appeared only in 3D. The malignancy is regulated at the level of tissue organization by the interaction between the cell and the extracellular matrix. It’s a sign that the tissue architecture governs the genome and that when it is damaged, as it is in the aging process, we become more susceptible to cancer,” the scientist said.
If her theory is correct, an intervention to correct the tissue architecture could make the cancer cells go back to behaving as normal cells. In fact, Bissell was able to prove this hypothesis.
The group observed that the surface of the malignant cells presented six times more integrins and seven times more EGFR than normal. By using an inhibitor of just one of these integrins, the disorderly cell growth was reversed.
“They continued to have the same aberrant genome, but the disordered growth was reversed because the structural architecture exercises a dominant influence over the genome. The phenotype exercises a dominant influence over the genotype, even in dealing with cancer,” Bissell added.
The role of actin
After three decades of investigation, Bissell believes that she is close to discovering the mechanisms through which communication between the mammary cell and the extracellular matrix occurs, thanks in part to collaboration with Brazilian researcher Alexandre Bruni-Cardoso, a faculty member at the Chemistry Institute of the University of São Paulo.
During his postdoctoral research, carried out at the Bissell Laboratory, Bruni-Cardoso helped explain how the protein actin is transported from inside to outside the cell nucleus.
“Actin is a protein that makes up the cytoskeleton. It is composed of fibers that help to give cells shape and movement. Over the past 30 years, studies have begun to point to the fact that actin is also found in the nucleus, and more recently, studies have shown that there it interacts with other nuclear proteins to regulate gene transcription,” Bruni-Cardoso explained.
In a previous study, also conducted at the Bissell Laboratory, postdoctoral fellow Virginia Spencer used a mouse mammary cell lineage to demonstrate that the higher the amount of actin found in the nucleus, the more the cells proliferated.
When treating the cell culture with the protein laminin – one of the most important proteins in the cells’ basal membrane – Spencer observed that the amount of actin in the nucleus fell drastically and that this occurred well before the cells stopped proliferating.
By repeating the experiment, but this time adding a peptide that stopped actin from exiting the nucleus, Spencer observed that the laminin’s inhibitory signals were annulled and that the cells continued to proliferate. The study was published in the Journal of Cell Science in 2011.
Using a human mammary cell culture as a model, Bruni-Cardoso began to investigate how the laminin signals were able to reach the actin in the nucleus.
“We identified the specific protein that receives the laminin signal, goes to the nucleus and exports the actin from the nucleus. The results should be published soon,” Bruni-Cardoso said.
In a tumor cell lineage, the researcher observed that this signaling is deregulated, and that even in the presence of laminin, the levels of actin in the nucleus do not stop growing.
“This may be one of the reasons why malignant cells proliferate uncontrollably. The discovery paves the way for the study of drugs that could correct the cell signaling,” Bruni-Cardoso noted.
According to Bissell, the actin appears to act as a switch in activating cell growth. “The question now is to find out exactly how it works. Today, we know that it is not only architecture but also signaling that determines cell behavior,” she concluded.
Republish
The Agency FAPESP licenses news via Creative Commons (CC-BY-NC-ND) so that they can be republished free of charge and in a simple way by other digital or printed vehicles. Agência FAPESP must be credited as the source of the content being republished and the name of the reporter (if any) must be attributed. Using the HMTL button below allows compliance with these rules, detailed in Digital Republishing Policy FAPESP.




